晶宇生物科技實業股份有限公司 DR. Chip Biotechnology Inc.
公司簡介
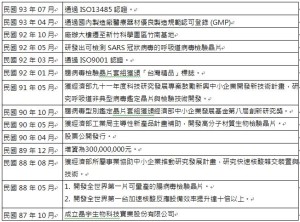
晶宇生物科技實業股份有限公司於1998年10月在工業技術研究中心成立,爲臨床診斷提供經濟實惠的高品質的生物醫學産品,致力於改進和簡化目前的生物研究法及臨床診斷工具來滿足消費者需求。晶宇至今已發展出三種科技平臺,製造了多種生物晶片産品、影像分析裝置、軟體和生物試劑,並在美國、歐洲、日本、澳洲、臺灣和中國等地申請了約五十件專利。2003年,晶宇將總部遷址於臺灣新竹科學工業園區竹南園區。站在生物晶片革命的最前沿,本公司建立了GMP標準工廠及ISO質量管理體系來滿足市場需求的高速增長,持續貫徹合理的計劃,從産品設計、生産到包裝都經過嚴格的控制、跟蹤和記錄,以確保生産出最優質的産品,並採用最新微流體和記憶裝置科技來發展處理型生物晶片體系,繼續爲病原體、遺傳和慢性疾病以及食物安全檢驗的分子診斷提供技術平臺。
爲進一步推動技術發展和産品商業化,晶宇目前在臺灣地區已和享受盛譽的研究所及公司進行合作、並在歐洲、澳洲結合許多合作夥伴,共同為生物晶片產業努力。
Company Profile
DR. Chip Biotech Inc. was founded at the Incubator Center of the Industrial Technology Research Institute (ITRI) in October 1998, with the goal to develop low cost and high quality bio-medical products for clinical diagnosis. We continue improving and simplifying current biological research methods and clinical diagnostic tools to meet the demands of our customers. We have developed three technological platforms and filed about fifty patents in the US, EU, Japan, Australia, Taiwan, and China. Today DR. Chip develops and manufactures several biochip products, image analysis device and software, and biological reagents. We adopts the state-of-the-art facilities and total quality management systems for manufacturing to meet the rapid expansion and increasing demands of the market. To stand at the forefront of the biochip revolution, we adopt latest microfluidics and MEMS technologies for developing lab-on-a-chip system.
DR. Chip currently collaborates with prestigious domestic research institutes and and many others in Europe, Australia, and great China.We are continuous applying our technology platform to the molecular diagnosis of pathogenic organisms, genetic and chronic diseases, and food safety inspection.



TKS
TKS